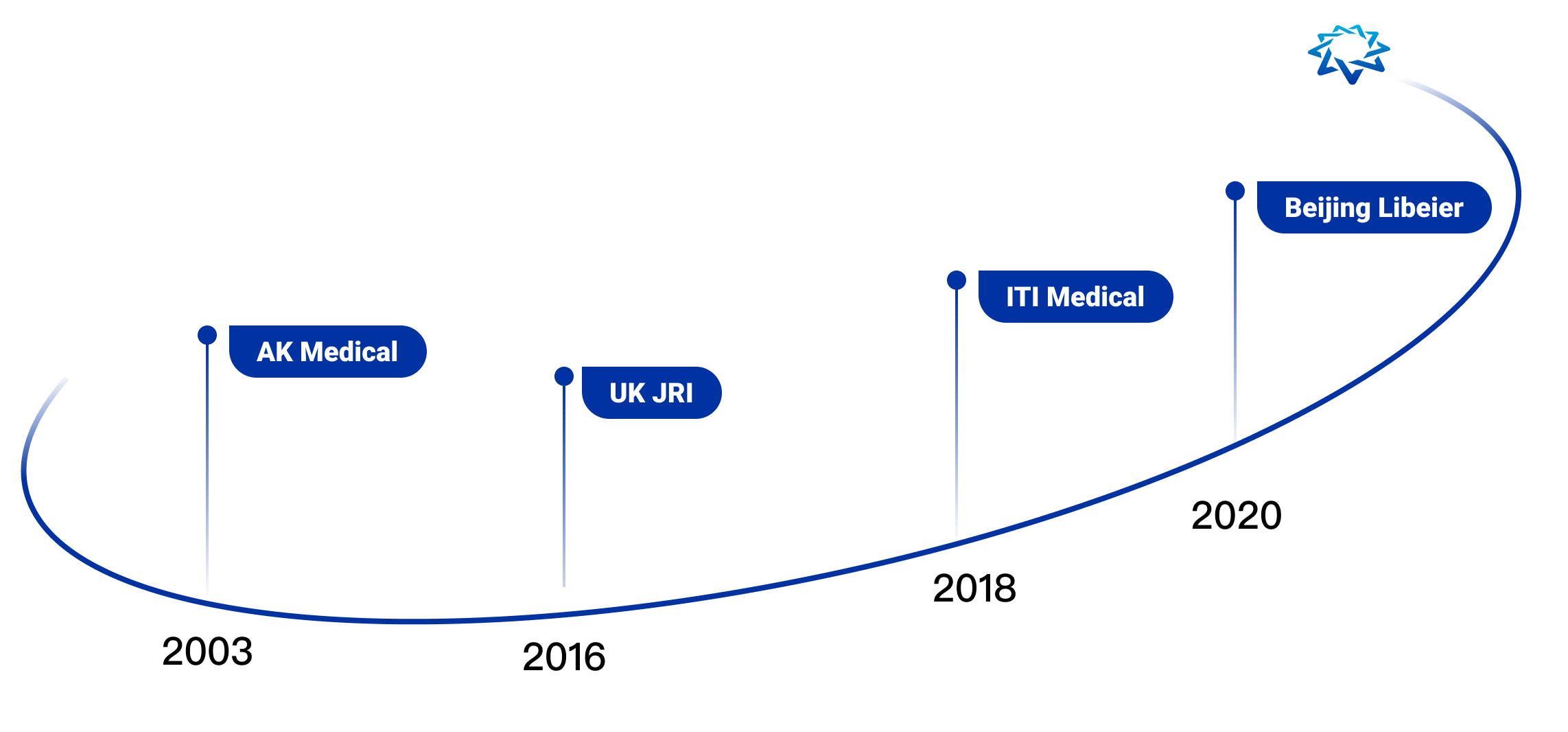
Founded in 2003, AK Medical Holdings Limited (AK Medical) is an international orthopedic industry group that combines research, product development, large-scale manufacturing and specialized marketing, and was listed on the main board of the Hong Kong Stock Exchange (01789.HK) in 2017.
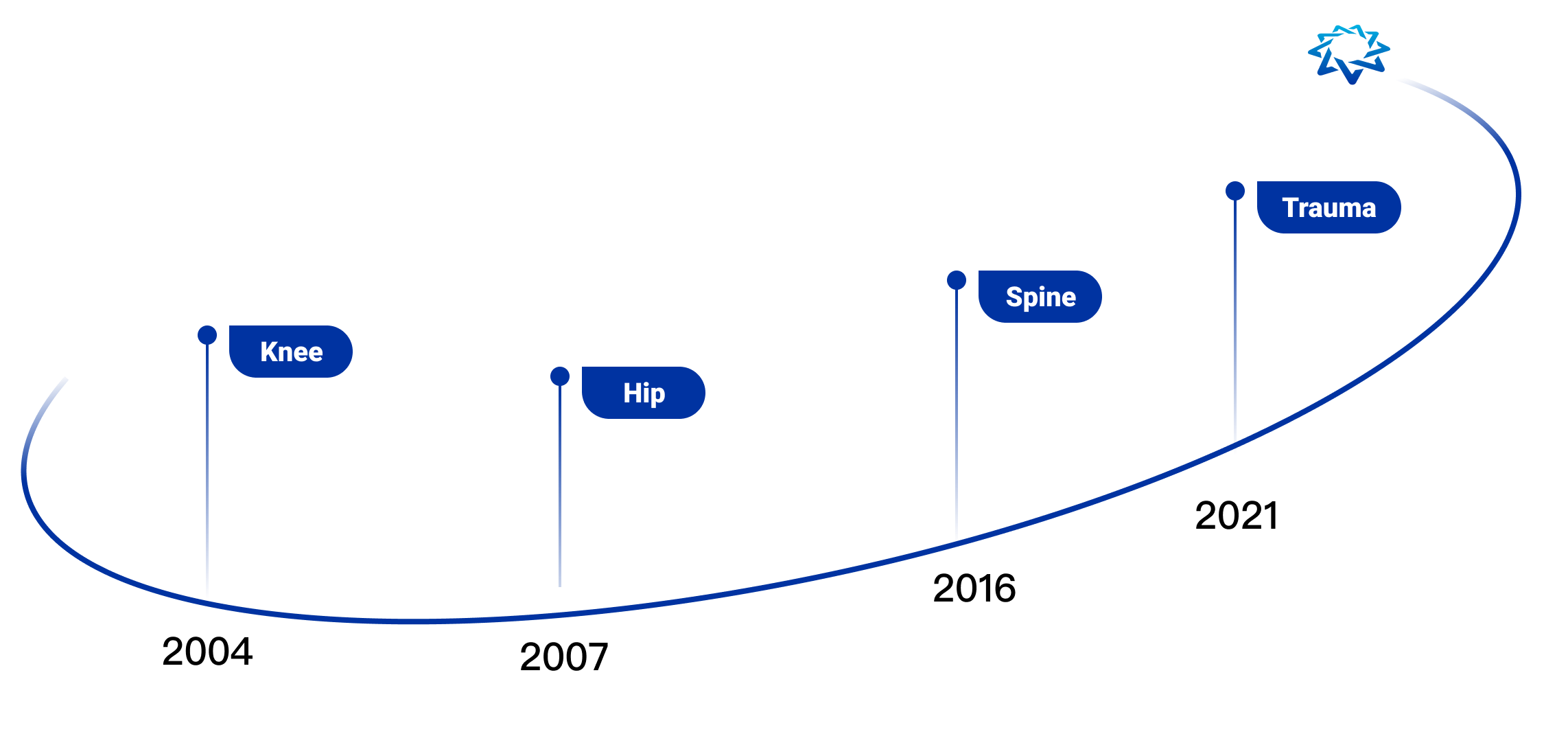
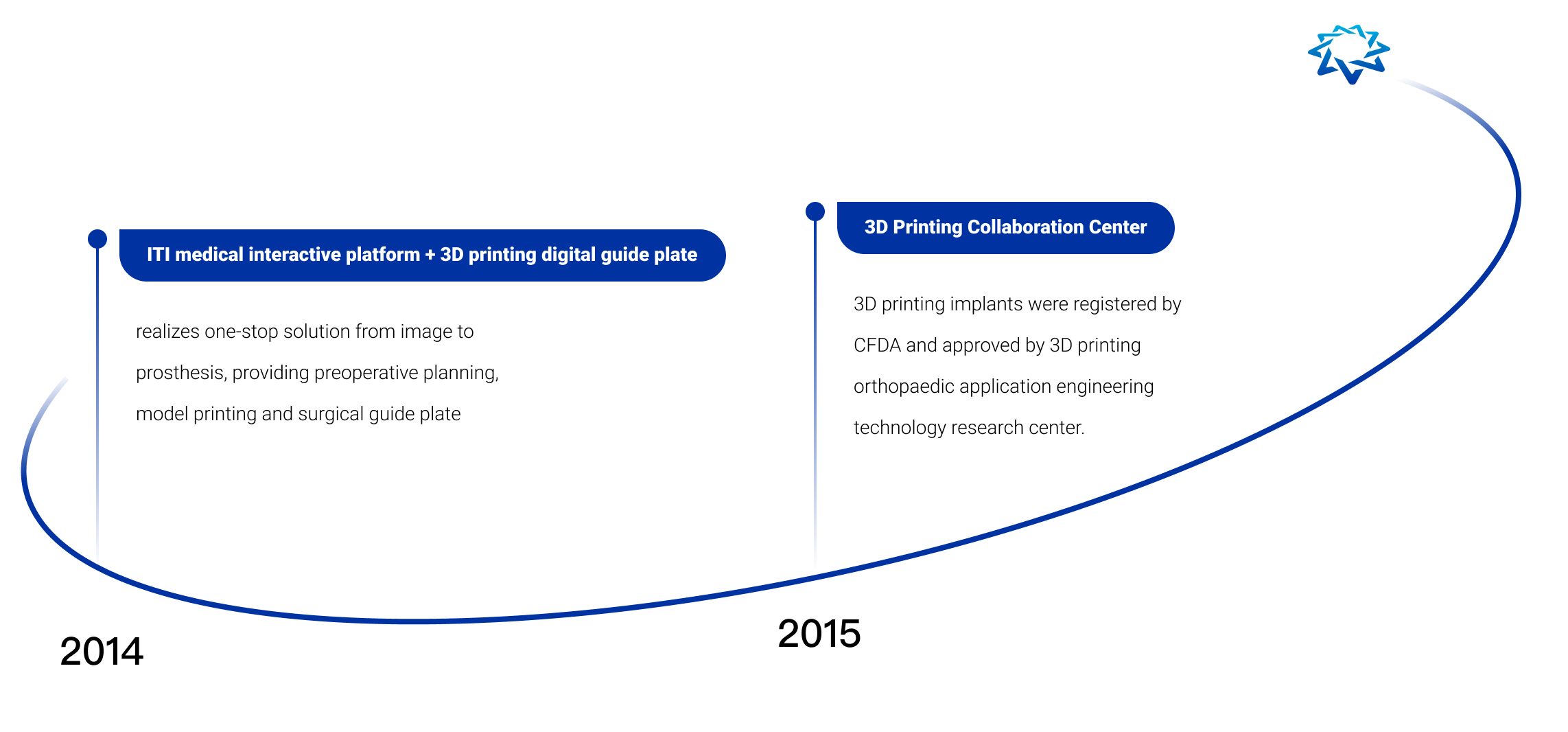
AK Medical is the first company in the world to commercialize and apply additive manufacturing technology (3D printing technology) to joint replacement, spine, trauma repair and other implants. We have a 3D printing precision building technology platform (3DACT), Supravit vacuum plasma spraying technology platform, One Point navigation/robotic intelligence technology platform, and the ICOS personalization platform.
At present, AK Medical is the leading enterprise in the field of artificial joints in China with the first market share, ranked No. 1 in terms of domestic market share and in the Top 10 for the global hip joint market. AK medical products are widely used in more than 7,500 medical institutions in over 40 countries and regions, including China, UK, Japan, Korea, Spain and South Africa. We have R&D centers in Beijing, Shanghai and the UK, and manufacturing bases in Changping, Beijing, Changzhou, Jiangsu and Sheffield, UK, to serve our global customers. On average, one AK prosthesis is implanted every minute.